As scientists, we feel motivated to better understand the complexities around us and make innovative changes in our ever-evolving world. The research done by Dr. Gerdon’s undergraduate students at Emmanuel College motivates and educates students in chemistry using a simple philosophy. First and foremost, our research aims to inspire students in chemical research by giving them hands-on instruction in a professional laboratory setting. Experimentation in novel areas of chemistry such as nanotechnology encourages students to explore their curiosities and gain experience in the field of research. Second to education, our researchers complete scientifically relevant experiments in three main fields of study: biomineralization, analytical biochemistry, and nanotechnology.
 Biomineralization refers to the process of mineralization taking place within nature. A multitude of examples can be found across the animal kingdom, but the process of biomineralization is still not well understood. Biomineralization can be seen on a macroscopic and microscopic level, whether it be the calcite formed by lobsters or the magnetite mineralized by bacteria. The ability of a living being to form mineral from its environment or its own body is often advantageous to its survival. Our research focuses on the biomineralization pathway of hydroxyapatite. This highly structured, crystalline calcium phosphate mineral is the main component of teeth and bones in mammals. Hydroxyapatite is largely responsible for the strength of teeth, as it’s highly ordered crystal lattice structure provides rigidity. In our quest to understand how hydroxyapatite is formed, we hope to eventually offer some insight into bone health and regeneration.
Biomineralization refers to the process of mineralization taking place within nature. A multitude of examples can be found across the animal kingdom, but the process of biomineralization is still not well understood. Biomineralization can be seen on a macroscopic and microscopic level, whether it be the calcite formed by lobsters or the magnetite mineralized by bacteria. The ability of a living being to form mineral from its environment or its own body is often advantageous to its survival. Our research focuses on the biomineralization pathway of hydroxyapatite. This highly structured, crystalline calcium phosphate mineral is the main component of teeth and bones in mammals. Hydroxyapatite is largely responsible for the strength of teeth, as it’s highly ordered crystal lattice structure provides rigidity. In our quest to understand how hydroxyapatite is formed, we hope to eventually offer some insight into bone health and regeneration.
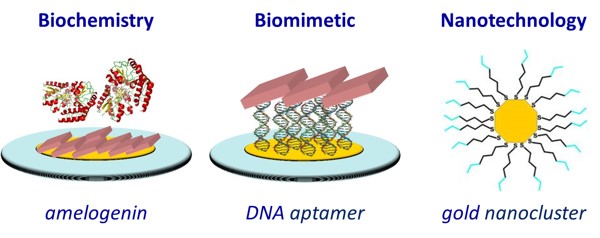
Besides directly studying mineral formation, biochemistry and biomimetic processes are used to better understand hydroxyapatite. By making use of the biochemical properties of hydroxyapatite, we can gain insight into its formation and functioning in a physiological setting. We focus our efforts on an extracellular matrix protein called amelogenin. This protein is involved with the formation of enamel and is believed to regulate the growth of hydroxyapatite. The biochemistry between hydroxyapatite and amelogenin enables us to learn more about hydroxyapatite in comparison with other calcium phosphate minerals.
Biomimetics, the imitation or modeling of systems found in nature, can also be used to study the formation and properties of hydroxyapatite. Scientists have benefited greatly from studying the unique skills organisms have developed over years simply by natural selection. By mimicking these skills, we can make use of them to learn more about the world around us. We use two biomimetic models in our research: DNA and nanoparticles. DNA is a molecule made up of nucleic acids that are held together by a negatively charged backbone. This backbone acts as a scaffold for the formation of hydroxyapatite. Similarly, we are using nanoparticles as a way to synthesize hydroxyapatite more efficiently. Nanoparticles are particles with a diameter anywhere between 1 and 100 nanometers. Both of these templates are a part of the larger field of biomimetic technology, which continues to be explored with great enthusiasm in our lab and beyond.
Our researchers gain valuable laboratory skills as they are exposed to a variety of different instrumental techniques. They learn how to conduct research in a professional laboratory setting, while contributing to the scientific community in a meaningful way. We learn to use a variety of instruments and analytical techniques such as microscopy, gel electrophoresis, spectroscopy, and more. Our researchers are even developing their own analytical methods to measure hydroxyapatite mineralization. Each different avenue of experimentation is helping to shed light on the process of biomineralization, in the hopes of contributing to biotechnological solutions to bone regeneration and repair.
Recent Comments