The Details – a technical introduction
Written by Sarah Faulkner ’15
Biomimetic materials, synthetic materials that mimic what biology produces, have been pursued for years to better understand how they can be applied to healthcare and biotechnology challenges. The application of these biomaterials face several daunting issues, often due to a fundamental lack of understanding of their basic functioning. Hydroxyapatite, (Ca5(PO4)3OH) is one such biomaterial. Hydroxyapatite is a highly ordered, crystalline mineral found mainly in tooth enamel and bone. The use of hydroxyapatite in biomedical technology requires knowledge of how to manipulate and influence the mineral’s formation and function. The finer details of biomineralization, the process by which mineral comes to form, are still largely unknown. If HAP is to be applicable in the field of biotechnology, there remains a great need to describe the process of its biomineralization. Controlling crystallinity and quantifying the kinetics of precipitation are key steps in broadening the use of HAP in the biomedical field. This will require a consortium of chemical techniques, including the analysis of mineral formation and the creation of templates to control various aspects of biomineralization.
Analysis
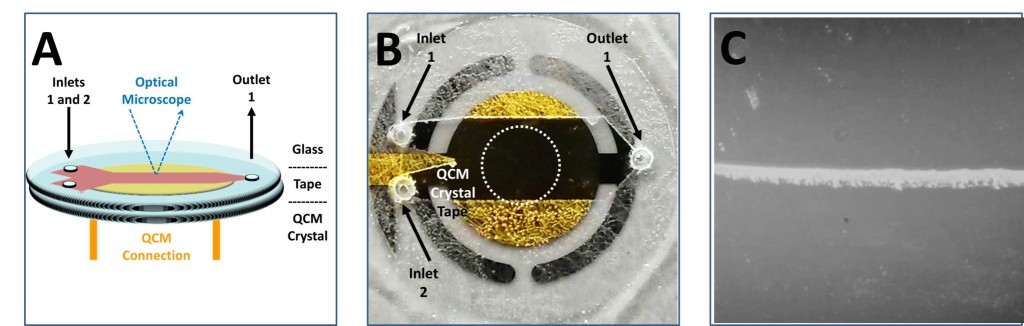
We have embarked on several projects, each taking a unique perspective on the aforementioned tasks. Using a quartz crystal microbalance (QCM), our researchers are utilizing real-time analytical methods to observe the kinetics of HAP mineralization and how it is affected by varying environmental conditions. Initial research confirmed our hypothesis that DNA, as an anionic polymer, can be utilized as a template for calcium phosphate mineralization (Ngourn et al). Our researchers were able to quantitatively determine a range of nucleation times and kinetic rates using QCM mass analysis. QCM is also being integrated with a microfluidic, multi-stream solution flow cell, designed and built by the students of the group (Conklin et al). These flow cells control how solution flows over the crystal in the QCM, thus controlling where and when mineralization can occur. Using this design, our researchers can ensure the mineral forms on the most sensitive part of the QCM thus securing a more accurate and complete understanding of the mineralization process. A combination of QCM mass analysis, optical microscopy, and laminar flow design make this an ideal set-up for studying HAP mineralization. These devices show great promise as an analytical system designed to study very controlled mineralization experiments, while decreasing the occurrence of non-specific mineralization.
[1] – Ngourn, S.C.; Butts, H.A.; Petty, A.R.; Anderson, J.A.; Gerdon, A.E. “Quartz Crystal Microbalance Analysis of DNA-Templated Calcium Phosphate Mineralization” Langmuir 2012, 28, 12151-12158.
[2] – Conklin, G.; Ngourn, S.C.; Gerdon, A.E. “Quartz crystal microbalance with microfluidic multi-stream solution control for mineralization kinetic analysis” Sens. Actuators B 2015, 214, 174-180.
(A) Diagram of microfluidic cell attached to commercial QCM quartz crystal and integrated with bright field optical microscopy.(B)Image of a 1 in QCM crystal with attached tape and glass, but no inlet/outlet tubing.2 The third image depicts laminar flow and mineral formation occurring in the sensitive region of the QCM.1
Nanotechnology
Templated mineralization of HAP using short-stranded DNA and gold nanoparticles is being studied by Dr. Gerdon and his research team. The gold nanoparticles are surrounded by a protective organic shell, classifying them as monolayer protected clusters (MPCs). This project aims to synthesize the nanoparticles while controlling their diameter and assessing their ability to act as a viable template for calcium phosphate mineralization. MPCs, with mixed monolayers of multiple functional ligands (DNA and uncharged polyethylene glycol), have demonstrated successful template-directed mineralization of calcium phosphate (Vasconcellos et al). We’ve shown that this combination of functional ligands allows for control and manipulation of solvated radii, steric effects, aggregation of particles, and overall charge density. Additionally, it has been shown that the gold cores of the MPCs confine a large number of protective ligands to a small volume. This inherently provides an increased negative effective local charge, encouraging the mineral formation. Using FT-IR and TEM data, our researchers have demonstrated successful templating and control of mineralization using MPCs functionalized with multiple ligands.
[3] – Vasconcellos, K.B.; McHugh, S.M.; Dapsis, K.J.; Petty, A.R.; Gerdon, A.E. “Biomimetic nanoparticles with polynucleotide and PEG mix-monolayers enhance calcium phosphate mineralization” J. Nanoparticle Res. 2013, 15, 1942.
TEM images of material mineralized with T-MPC template after 2 h. a) A variety of particle sizes are observed showing significant polydispersity. b) A large crystalline particle showing plate- and needle-like formations and entrapped MPCs. c) Two small and newly forming particles with MPC templates either entrapped or adhering to growing edges.3
DNA
The use of short, single-stranded DNA as a template for the biomineralization of HAP is also being studied. The negatively charged phosphate backbone of DNA binds with the positively charged calcium cation, acting as a scaffold for the formation of the calcium phosphate mineral (Ngourn et al). A library of 10^14 unique DNA strands is assessed with a process known as SELEX – Systematic Evolution of Ligands by Exponential Enrichment. The strands of DNA undergo several rounds of increasingly stringent conditions. Each round of evolution theoretically eliminates weak templates and amplifies the strong ones. Two different properties of DNA are examined in this study – binding affinity and mineralization strength. QCM is used to quantitatively analyze the kinetics of mineralization and observe the evolution process in real-time. Our researchers have observed the evolution of the library using salt levels, mineral formation, and overall binding as a means to increase stringency over the course of several rounds. This project will culminate by analyzing progress over the course of several rounds and eventually sequencing the resulting strands.
[1] – Ngourn, S.C.; Butts, H.A.; Petty, A.R.; Anderson, J.A.; Gerdon, A.E. “Quartz Crystal Microbalance Analysis of DNA-Templated Calcium Phosphate Mineralization” Langmuir 2012, 28, 12151-12158.
Amelogenin
Proteins act as templates for mineralization in vivo. Amorphous calcium phosphate (ACP), a mineral precursor to HAP, has been studied along-side HAP by our group using amelogenin as a protein template. The real-time, analytical advantage of QCM is used to measure mineral and protein interaction between HAP or ACP and amelogenin. We hypothesize that amelogenin will have different binding characteristics between ACP and HAP and are pursuing these unique studies.
Undergraduate Student Success
By contributing to this research, the students involved learn a variety of valuable laboratory techniques and instruments: optical microscopy, PCR, gel electrophoresis, QCM, FT-IR, Raman spectroscopy, and more. While contributing to the myriad of laboratory skills undergraduate students acquire during their time at college, this research group provides a place for students to practice their learned abilities in a professional laboratory setting. Students of this research group are coming to understand the properties of HAP, the process by which it biomineralizes, and the means to control mineralization using a variety of templates. Using a unique union of biochemistry, materials chemistry, and physics, our researchers are tirelessly working to understand and influence the formation of this powerful mineral found in our bones.

Recent Comments